Redesign and engineering of a dioxygenase targeting biocatalytic synthesis of 5-hydroxyl leucine†
Abstract
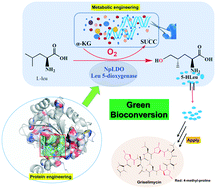
5-Hydroxyl leucine (5-HLeu) is a promising precursor for the biosynthesis of anti-tuberculosis griselimycins. Rate limiting steps in the biosynthesis of 5-HLeu include the catalytic activity of the enzyme responsible for its biosynthesis, leucine dioxygenase (NpLDO), and an insufficient supply of the cosubstrate α-ketoglutarate (α-KG). These limitations can potentially be overcome by developing a commercial microbial cell factory that synthesizes 5-HLeu efficiently. In this report, a protein engineering strategy was used to enhance the catalytic activity of NpLDO. A highly active mutant (MT3) was obtained that displayed a 4.7-fold increase in catalytic activity when compared with that of the wild-type enzyme. The efficient biosynthesis of 5-HLeu was further optimized using a metabolic engineering strategy involving gene editing of the TCA cycle (ΔsucA and ΔaceA), improvement of the metabolic flux (gltA and icd), and the introduction of heterologous glutamate oxidase and catalase to enhance the α-KG supply. The yields of 5-HLeu reached 5.638 g L−1 in engineered E. coli MG1655 cells, making this the first report describing the microbial biosynthesis of 5-HLeu.


 Please wait while we load your content...
Please wait while we load your content...