Computational insights into different chemoselectivities in Rh2(ii)-catalyzed N-aryl nitrene and analogous Rh2(ii)/Cu(i)-catalyzed aryl-substituted carbene involving reactions†
Abstract
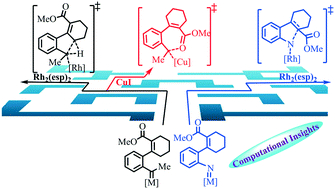
Computational studies were carried out to investigate the mechanisms and the origins of chemoselectivities among multiple reactive sites for Rh2(II)-catalyzed N-aryl nitrene and analogous Rh2(II)/Cu(I)-catalyzed aryl carbene involving reactions. For the reaction of Rh2(II)-catalyzed ortho-alkenyl substituted aryl azides to produce 3H-indoles, after the formation of the crucial Rh2(II)-N-arylnitrene intermediate, the computational results suggest that it is more advantageous for the triplet Rh2(II)-N-arylnitrene to undergo intramolecular nitrene addition to the alkenyl moiety followed by the formation of an N-heterocycle in the singlet state. Driven by re-aromatization, the subsequent migration of the ester group could proceed to afford the final 3H-indole derivative product. For the mechanism of the Rh2(II)-catalyzed analogous aryl-substituted carbene transfer reaction, calculation results show that it is favorable for the key Rh2(II)-arylcarbene intermediate to trigger intramolecular allyl sp3 C–H insertion thereby forming the 1H-indene derivative in a concerted manner. The nucleophilic attack of the alkenyl group to the carbene moiety is unfavorable mainly due to the steric hindrance between the ester group and the methyl group attached to the carbene moiety. In addition, for the Cu(I)-catalyzed carbene transfer reaction with the same substrate, after the formation of the key Cu(I)-carbenoid intermediate, the nucleophilic attack of the oxygen atom of the carbonyl group to the carbene moiety is found to be the most favorable pathway, yielding an epoxide intermediate through subsequent cyclization. Afterward, ring opening of the three-membered ring and methyl migration can follow to give the final α-alkoxy 2H-naphthalenone product. Compared with the Rh2(II)-catalyzed counterpart, the steric hindrance for the O attack pathway is negligible in the Cu(I)-catalyzed reaction due to the absence of the bulky ligand.


 Please wait while we load your content...
Please wait while we load your content...