In situ XAS study of the local structure and oxidation state evolution of palladium in a reduced graphene oxide supported Pd(ii) carbene complex during an undirected C–H acetoxylation reaction†
Abstract
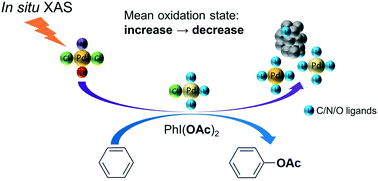
In situ X-ray absorption spectroscopy (XAS) investigations have been performed to provide insights into the reaction mechanism of a palladium(II) catalyzed undirected C–H acetoxylation reaction in the presence of an oxidant. A Pd(II) N-heterocyclic carbene complex π-stacked onto reduced graphene oxide (rGO) was used as the catalyst. The Pd speciation during the catalytic process was examined by XAS, which revealed a possible mechanism over the course of the reaction. Pd(II) complexes in the as-synthesized catalyst first go through a gradual ligand substitution where chloride ions bound to Pd(II) are replaced by other ligands with a mean bond distance to Pd matching Pd–C/N/O. Parallel to this the mean oxidation state of Pd increases indicating the formation of Pd(IV) species. At a later stage, a fraction of the Pd complexes start to slowly transform into Pd nanoclusters. The mean average oxidation state of Pd decreases to the initial state at the end of the experiment which means that comparable amounts of Pd(0) and Pd(IV) are present. These observations from heterogeneous catalysis are in good agreement with its homogeneous analog and they support a Pd(II)–Pd(IV)–Pd(II) reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...