Asymmetric ring opening of racemic epoxides for enantioselective synthesis of (S)-β-amino alcohols by a cofactor self-sufficient cascade biocatalysis system†
Abstract
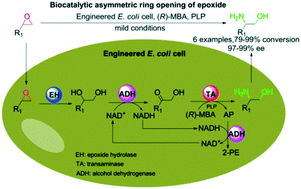
A novel one-pot epoxide hydrolase/alcohol dehydrogenase/transaminase cascade process for the asymmetric ring opening of racemic epoxides to enantiopure β-amino alcohols is reported. The product (S)-β-amino alcohols were obtained in 97–99% ee and 79–99% conversion from readily available racemic epoxides.
- This article is part of the themed collection: Asymmetric catalysis


 Please wait while we load your content...
Please wait while we load your content...