Prominent role of mesopore surface area and external acid sites for the synthesis of polyoxymethylene dimethyl ethers (OME) on a hierarchical H-ZSM-5 zeolite†
Abstract
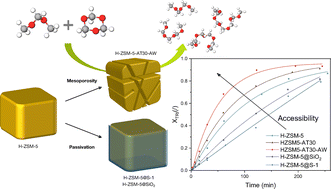
H-ZSM-5 zeolite has been shown to be an active catalyst for the synthesis of polyoxymethylene dimethyl ethers (OME). However, we demonstrated – by passivation of the zeolite's external surface – that the reaction rate is limited due to severe internal diffusion limitations of the reactants and products. External acid sites thus played a more prominent role in the observed overall reaction rate compared to the acid sites in the zeolite's micropores. Through controlled introduction of an intercrystalline network of mesopores the zeolite's activity was significantly enhanced by allowing a more significant part of the reaction to take place within the zeolite's micropores. By optimising alkaline treatment and consequent acid wash of H-ZSM-5, we achieved a two-fold increase in the initial reaction rate and a 10% increase in selectivity towards OME with 3 to 5 oxymethylene units (OME3–5), which are the more desirable products.


 Please wait while we load your content...
Please wait while we load your content...