The importance of direct reduction in the synthesis of highly active Pt–Sn/SBA-15 for n-butane dehydrogenation†
Abstract
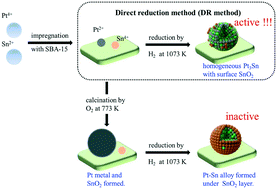
Supported Pt–Sn bimetallic catalysts directly reduced by H2 (DR method) are highly active for the dehydrogenation of n-butane, while the catalysts calcined in air, followed by H2 reduction (CR method), are totally inactive regardless of the calcination temperature and Sn/Pt ratio. The present work investigated the detailed bulk and surface structural properties of the catalysts with various characterization techniques. The results suggest that both DR and CR methods led to the formation of bulk Pt–Sn alloys (e.g. Pt3Sn and PtSn). However, calcination before reduction significantly changed the catalyst surface properties. The SnO2 layer was located on the surface of the Pt–Sn alloys and/or Pt nanoparticles when the catalysts were prepared with the CR method. In contrast, the DR method induced more surface exposed Pt atoms from the Pt3Sn alloy, which played an important role in the superior catalytic behaviors for n-butane dehydrogenation.


 Please wait while we load your content...
Please wait while we load your content...