Ligand-functionalized Pt nanoparticles as asymmetric heterogeneous catalysts: molecular reaction control by ligand–reactant interactions†
Abstract
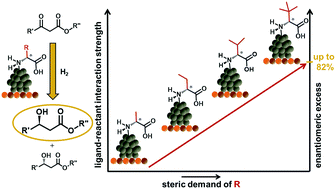
The asymmetric hydrogenation of β-keto esters over platinum nanoparticles (NPs) functionalized with different α-amino acids was explored. By systematic variation of the structural complexity of the functionalizing ligands, we determined structure–selectivity relationships and further improved our understanding of ligand–reactant interactions. We identified attractive interactions between the lipophilic substituents of ligands and reactants as one of the major contributing factors governing the mutual orientation of ligands and reactants and, thus, the stereoselectivity of the catalytic reaction. For the first time, an enantiomeric excess (ee) above 80% is reported for supported ligand-functionalized NPs, which demonstrates the potential of these materials as a novel type of asymmetric heterogeneous catalyst. Our results reveal that the molecular principles employed in homogeneous catalysis can be utilized to achieve stereoselective catalytic reactions even on rather non-uniform surfaces like small NPs. This opens up yet unexplored possibilities for manipulating reactions on catalytic surfaces to control selectivity.
- This article is part of the themed collection: 2018 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...