Formaldehyde–isobutene Prins condensation over MFI-type zeolites†
Abstract
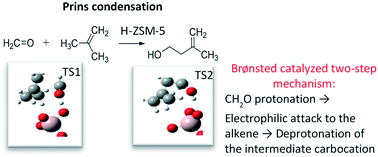
The liquid phase Prins condensation of formaldehyde with butenes on H-ZSM-5 (MFI) zeolite catalysts was investigated showing that reaction rates follow the order isobutene > 1-butene > cis-2-butene. The catalytic rates for medium-pore zeolite H-ZSM-5 were not only a larger than for H-beta, but also selective for 3-methyl-3-buten-1-ol during formaldehyde condensation with isobutene. Isoprene forms, under the optimal reaction conditions, either in a second dehydration step of the unsaturated alcohol, or in a single step over H-ZSM-5 with optimal Si/Al ratio (Si/Al = 40). Mechanistic studies revealed that sequential Prins cyclization and hetero-Diels–Alder reactions of the desired products, forming six carbon species, occur only at slow rates over H-ZSM-5. Using DFT methods it was determined that the reaction follows a two-step mechanism: protonation of formaldehyde and electrophilic attack to the alkene, and deprotonation of the resulting intermediate carbocation. For isobutene and 1-butene, the electrophilic addition is coordinated with the protonation of the formyl group, but for cis-2-butene, it is coordinated with the proton back-donation step. The rate-limiting step switches from electrophilic addition for isobutene to proton elimination for the other two C4 isomers.


 Please wait while we load your content...
Please wait while we load your content...
