Unveiling the mechanisms and secrets of chemoselectivities in Au(i)-catalyzed diazo-based couplings with aryl unsaturated aliphatic alcohols†
Abstract
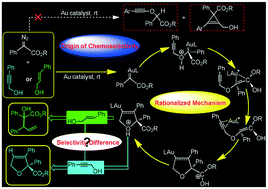
Density functional theory (DFT) calculations have been conducted to unravel the mechanisms and chemoselectivities of Au-catalyzed diazo-based couplings with phenyl unsaturated aliphatic alcohols: the propargyl alcohol Ba resulting in the [4 + 1]-cycloaddition product P4a and the allyl alcohol Db giving the [2,3]-σ rearrangement species P5b. P4a formation involves a catalyst interaction with phenyldiazoacetate, N2 release, a hydroxyl O nucleophilic attack of Ba, a [1,4]-H shift, coordination isomerization, 5-endo-dig cyclization, a [4,1]-H shift and a H2O-assisted [1,3]-H shift. After the [4,1]-H shift, the slightly less favorable five-membered ring-opening possibly follows to afford trace P5a ([2,3]-σ rearrangement product), which would be kept in solution due to subsequent irreversible evolution. In addition, the Ba-involved chemoselectivity was probed and explained as follows: (i) both large H(hydroxyl)⋯C(carbene) electrostatic repulsion and strong three-membered ring strain involved in the TS make the formation of the O–H insertion product P1a difficult and (ii) the nucleophilic attack from the C2 atom of Ba brings about a structural twisting and thus increases the energy penalty forming the cyclopropenation product P2a. On the other hand, compared with the sp-C2 atom of Ba, the sp2-C2 atom of Db greatly facilitates the five-membered ring-opening step because of the presence of an extra pπ–pπ orbital overlap and eventually provides P5b exclusively.


 Please wait while we load your content...
Please wait while we load your content...