Facile synthesis of amorphous mesoporous manganese oxides for efficient catalytic decomposition of ozone†
Abstract
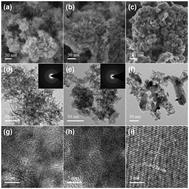
Amorphous mesoporous manganese oxides (MnOx) with different microstructures were synthesized via a facile redox method between manganese acetate and potassium permanganate by modulating the addition sequence of the precursors and directly used for catalytic decomposition of ozone. The as-prepared catalysts were characterized by N2 physisorption, SEM, TEM, XRD, Raman, H2-TPR, O2-TPD, TGA, and XPS. Amorphous mesoporous MnOx had more surface oxygen vacancies and larger specific surface area than calcined MnOx with high crystallinity and exhibited superior ozone decomposition activity at room temperature. Kinetic study and characterization results revealed that the lower oxidation state of Mn resulting from mixed Mn2+, Mn3+ and Mn4+ in amorphous mesoporous MnOx was more favorable for activating ozone towards high initial ozone conversion. Moreover, the catalytic stability of amorphous mesoporous MnOx strongly depended on the relative humidity, and the elevation of the oxidation state and the accumulation of surface adsorbed water molecules possibly led to the catalyst deactivation. These results may shed new light on the design of novel amorphous manganese-based catalysts for ozone decomposition.


 Please wait while we load your content...
Please wait while we load your content...