Isobutane aromatization over a complete Lewis acid Zn/HZSM-5 zeolite catalyst: performance and mechanism†
Abstract
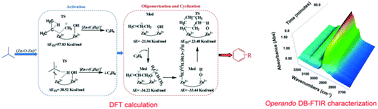
In short-chain alkane aromatization catalyzed by metal-modified HZSM-5, the metal cations acting as Lewis acid sites are considered to be the active centres for the dehydrogenation. However, other possible effects of the metal cations during the reaction have been neglected. In this study, a combination of experiments and DFT calculations has been carried out to investigate the complete effects of the Zn on ZSM-5 in isobutane aromatization. Zn8.47/HZSM-5 (Zn/Al > 1) with weak acidic properties was prepared as a Lewis acid sites-dominated catalyst. It was found that the (Zn–O–Zn)2+ Lewis acid sites acted as the main active sites and no bridged hydroxyl groups (Brønsted acid sites) were present. In isobutane conversion, it was surprisingly found that Zn8.47/HZSM-5 showed better catalytic performance at low temperature than HZSM-5 (Brønsted acid sites-dominated catalyst) and Zn2.34/HZSM-5 (Brønsted and Zn-Lewis acid sites co-existing catalyst). DFT calculations and operando dual-beam Fourier transform infrared spectrometry (DB-FTIR) characterization were employed to understand how the isobutane aromatization could be catalyzed on the Lewis acid site (Zn–O–Zn)2+ exclusively without the participation of bridged hydroxyl groups. It was directly observed over DB-FTIR that the Lewis acid-type Zn/HZSM-5 enjoyed a faster aromatization rate and higher stability than HZSM-5 under real aromatization conditions. This firstly testified that the complete reaction of isobutane aromatization could be exclusively catalyzed by (Zn–O–Zn)2+ Lewis acid sites without the participation of bridged hydroxyl groups. The [Zn-(OH)−]+ species could be produced in situ and acted as the Brønsted acid sites. Owing to the weak acidity of [Zn-(OH)−]+, the Lewis acid sites played a key role during the aromatization reactions via the carbanionic mechanism.


 Please wait while we load your content...
Please wait while we load your content...