An artificial metalloenzyme for carbene transfer based on a biotinylated dirhodium anchored within streptavidin†
Abstract
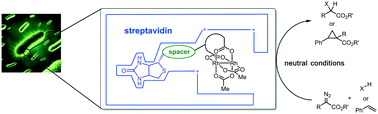
We report on artificial metalloenzymes that incorporate a biotinylated dirhodium core embedded within engineered streptavidin (Sav hereafter) variants. The resulting biohybrid catalyzes the carbene insertion in C–H bonds and olefins. Chemical- and genetic optimization allows to modulate the catalytic activity of the artificial metalloenzymes that are shown to be active in the periplasm of E. coli (up to 20 turnovers).

 Please wait while we load your content...
Please wait while we load your content...