Sea coral-like NiCo2O4@(Ni, Co)OOH heterojunctions for enhancing overall water-splitting†
Abstract
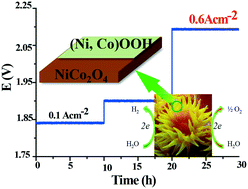
It is highly challenging to develop efficient and low-cost catalysts to meet the stringent requirements for high current density for industrial water electrolysis applications. We developed sea coral-like NiCo2O4@(Ni, Co)OOH heterojunctions, synthesized based on an epitaxial in-grown method using poly(ethylene glycol) (PEG) as a template, and explored them as efficient electrocatalysts for water-splitting. A two-electrode based alkaline electrolyzer was fabricated using NiCo2O4@(Ni, Co)OOH||NiCo2O4@(Ni, Co)OOH, which achieved a current density value of 100 mA cm−2 with a low potential of 1.83 V and a high current density which approached 600 mA cm−2 at a potential of 2.1 V along with strong stability. These are superior to most of the reported data for electrocatalysts operating at high current densities. In situ calculations based on density function theory reveal the occurrence of water-splitting on the NiCo2O4@(Ni, Co)OOH heterojunction surface. First-principles molecular dynamics simulations reveal that the stretching vibrations of metallic bonds of NiCo2O4@(Ni, Co)OOH heterojunctions open the hydrogen bonds of water. Understanding the mechanism of water-splitting at the heterojunctions from in situ theoretical calculations is helpful when developing new generation industrial catalysts.


 Please wait while we load your content...
Please wait while we load your content...