Catalytic transfer hydrogenation of biomass-derived 5-hydroxymethylfurfural into 2,5-bis(hydroxymethyl)furan over tunable Zr-based bimetallic catalysts†
Abstract
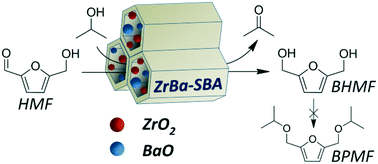
It is challenging to selectively convert highly active 5-hydroxymethylfurfural (HMF) into 2,5-bis(hydroxymethyl)furan (BHMF), a promising biomass-derived building block. In this work, a series of SBA-15 supported Zr-based catalysts were prepared and tested for the catalytic transfer hydrogenation of HMF into BHMF through the Meerwein–Ponndorf–Verley (MPV) reduction. A HMF conversion of 98.3% with a satisfactory BHMF selectivity of up to 92.2% was obtained at 150 °C in 2.5 h over ZrBa-SBA using isopropanol as the H-donor. It was shown that the total amount of acid sites of ZrBa-SBA considerably decreased with the introduction of BaO. In particular, the Brønsted acid sites of the catalysts became negligible, thus the etherification of HMF or BHMF could be completely suppressed over ZrBa-SBA. However, the remaining acid sites (mainly Lewis acid sites) of ZrBa-SBA were still sufficient for the MPV reduction of HMF, which enabled a high selectivity towards BHMF. Furthermore, ZrBa-SBA was found to be a robust catalyst that could be continuously used at least five times without significant loss of its catalytic activity.


 Please wait while we load your content...
Please wait while we load your content...