Elucidating the reactivity of methoxyphenol positional isomers towards hydrogen-transfer reactions by ATR-IR spectroscopy of the liquid–solid interface of RANEY® Ni†
Abstract
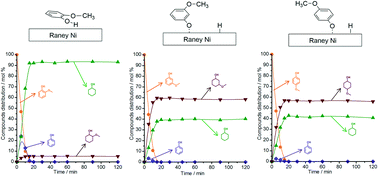
In the valorisation of lignin, the application of catalytic hydrogen transfer reactions (e.g. in catalytic upstream biorefining or lignin-first biorefining) has brought a renewed interest in the fundamental understanding of hydrogen-transfer processes in the defunctionalisation of lignin-derived phenolics. In this report, we address fundamental questions underlining the distinct reactivity patterns of positional isomers of guaiacol towards H-transfer reactions in the presence of RANEY® Ni and 2-PrOH (solvent and H-donor). We studied the relationship between reactivity patterns of 2-, 3- and 4-methoxyphenols and their interactions at the liquid–solid interface of RANEY® Ni as probed by attenuated total reflection infrared (ATR-IR) spectroscopy. Regarding the reactivity patterns, 2-methoxyphenol or guaiacol is predominantly converted into cyclohexanol through a sequence of reactions including demethoxylation of 2-methoxyphenol to phenol followed by hydrogenation of phenol to cyclohexanol. By contrast, for the conversion of the two non-lignin related positional isomers, the corresponding 3- and 4-methoxycyclohexanols are the major reaction products. The ATR-IR spectra of the liquid–solid interface of RANEY® Ni revealed that the adsorbed 2-methoxyphenol assumes a parallel orientation to the catalyst surface, which allows a strong interaction between the methoxy C–O bond and the surface. Conversely, the adsorption of 3- or 4-methoxyphenol leads to a tilted surface complex in which the methoxy C–O bond establishes no interaction with the catalyst. These observations are also corroborated by a smaller activation entropy found for the conversion of 2-methoxyphenol relative to those of the other two positional isomers.


 Please wait while we load your content...
Please wait while we load your content...