Investigating Saccharomyces cerevisiae alkene reductase OYE 3 by substrate profiling, X-ray crystallography and computational methods†
Abstract
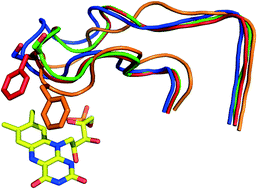
Saccharomyces cerevisiae OYE 3 shares 80% sequence identity with the well-studied Saccharomyces pastorianus OYE 1; however, wild-type OYE 3 shows different stereoselectivities toward some alkene substrates. Site-saturation mutagenesis of Trp 116 in OYE 3 followed by substrate profiling showed that the mutations had relatively little effect, opposite to that observed previously for OYE 1. The X-ray crystal structures of unliganded and phenol-bound OYE 3 were solved to 1.8 and 1.9 Å resolution, respectively. Both structures were nearly identical to that of OYE 1, with only a single amino acid difference in the active site region (Ser 296 versus Phe 296, part of loop 6). Despite their essentially identical static X-ray structures, molecular dynamics (MD) simulations revealed that loop 6 conformations differed significantly in solution between OYE 3 and OYE 1. In OYE 3, loop 6 remained nearly as open as observed in the crystal structure; by contrast, loop 6 closed over the active site of OYE 1 by ca. 4 Å. Loop closure likely generates a greater number of active site protein contacts for substrate bound to OYE 1 as compared to OYE 3. These differences provide an explanation for the differing stereoselectivities of OYE 3 and OYE 1, despite their nearly identical X-ray crystal structures.


 Please wait while we load your content...
Please wait while we load your content...
