Dimethyl ether carbonylation to methyl acetate over highly crystalline zeolite seed-derived ferrierite†
Abstract
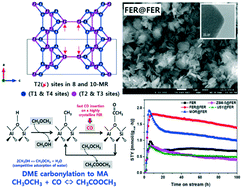
Gas-phase carbonylation of dimethyl ether (DME) to methyl acetate (MA) was investigated on ferrierite (FER) zeolite having different Si/Al molar ratios of 10.4–12.5 as well as high crystallinity synthesized by using various zeolite seed materials such as MOR, ZSM-5 and USY. The enhanced crystallinity of the FER prepared by simply using FER seeds (denoted as FER@FER) having newly formed mesopore structures was responsible for an increased amount of active Brønsted acid sites, which resulted in a higher MA productivity of 2.94 mmol gcat h−1 with MA selectivity of above 99%. The highly crystalline FER@FER revealed the suppressed deposition of aromatic coke precursors due to the presence of fewer defect sites. Compared to other zeolite seed-derived FER zeolites, the lesser amount of defect sites (extra-framework Lewis acid Al species, EFAL) on the FER@FER was successfully controlled through a recrystallization process. The active Brønsted acid sites for the DME carbonylation reaction mainly originated from the preferential formation of stable tetrahedral Al sites (especially the T2 sites of the Al–O–Si–O–Al framework of FER) on the 8- and 10-membered ring channels of the FER@FER. On those stable T2 sites having proper acid strength, the adsorbed methyl intermediates formed by the dissociation of DME can be transformed to acetyl adsorbates by a relatively faster CO insertion rate on the vicinal Brønsted acid sites, which results in a high catalytic stability and activity of the highly crystalline FER@FER.


 Please wait while we load your content...
Please wait while we load your content...