Efficient catalysts for oxygen evolution derived from cobalt-based alloy nanochains†
Abstract
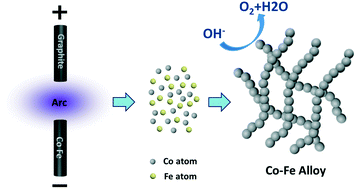
One-dimensional nanomaterials are widely used in electrocatalysis owing to their high charge transfer efficiency. In this work, Co–Fe alloy nanochains are presented as efficient catalysts for the oxygen evolution reaction. The nanochains with uniform nanospheres coupled to each other can range up to several micrometers. A metal–metal oxide heterostructure is attained when the alloy is exposed to the air or oxidized by the electrochemical process. The metal can inject electrons into the surface oxide, which changes the work function of the oxide and improves its catalytic efficiency. Series of samples with different compositions (Co, Co7Fe3, Co5Fe5, Co3Fe7, and Fe) were prepared and the effect of Fe/Co ratio on the catalytic activity was studied. Co7Fe3 exhibits the optimal performance with an onset point of 1.50 V (vs. RHE) and an overpotential of 365 mV at 10 mA cm−2. The nanochains also exhibit excellent stability with 92.0% current retention after a long-term chronoamperometry test. Cobalt-based alloys with other metals (Ti, Nb, and Mo) are synthesized by the same method and they have also shown promising application in the oxygen evolution reaction.


 Please wait while we load your content...
Please wait while we load your content...