Ten-fold boost of catalytic performance in thiol–yne click reaction enabled by a palladium diketonate complex with a hexafluoroacetylacetonate ligand†
Abstract
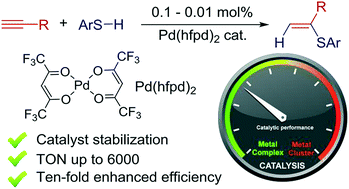
Palladium complexes with fluorinated acetylacetonate chelating ligands were studied as catalysts for alkyne hydrothiolation. A ten-fold increase in the catalytic efficiency was achieved by using 0.1 mol% of Pd(hfpd)2 complex (hfpd = hexafluoroacetylacetonate) with a variety of thiol–yne coupling partners. The principal possibility of a hundred-fold increase in the efficiency of Pd-catalyzed Markovnikov-type RSH addition with 0.01 mol% of the catalyst was successfully achieved with the hfpd ligand for the first time. The hexafluoroacetylacetonate chelating ligand not only enhanced the affinity of palladium centers to the triple bond of acetylene, but also stabilized the catalytic system against formation of insoluble polymeric [Pd(SPh)2]n species, thus ensuring that the reaction operates homogeneously. Utilizing other diketonate ligands resulted in cocktail-type catalysis with variable and poorly predictable contributions of homogeneous and heterogeneous pathways.


 Please wait while we load your content...
Please wait while we load your content...