Tunable Prussian blue analogues for the selective synthesis of propargylamines through A3 coupling†
Abstract
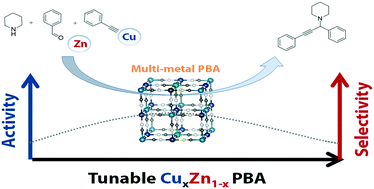
M1[Co(CN)6]2/3-type Prussian blue analogues (M1–Co PBAs) were studied as catalysts for the synthesis of propargylamines via A3 coupling of phenylacetylene, benzaldehyde and piperidine. Cu0.86Zn0.14–Co PBA was the best catalyst for the reaction by combining the high conversion obtained with Cu–Co PBA with the excellent selectivity obtained with Zn–Co PBA.


 Please wait while we load your content...
Please wait while we load your content...