Efficient C–C coupling of bio-based furanics and carbonyl compounds to liquid hydrocarbon precursors over lignosulfonate derived acidic carbocatalysts†
Abstract
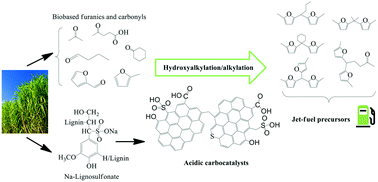
This paper demonstrates the catalytic potential of novel Na-lignosulfonate (LS) derived meso/macroporous solid protonic acids upon C–C coupling of bio-based furanics and carbonyl compounds. The materials demonstrated catalytic activity for solventless hydroxyalkylation/alkylation (HAA) of 2-methylfuran with furfural, acetone, butanal, cyclohexanone, levulinic acid and α-angelica lactone under mild reaction conditions (50–60 °C) producing branched-chain C12–C16 hydrocarbon precursors in yields approaching 96%. Moreover, the carbon materials exhibiting high total acidity (6–6.4 mmol g−1) outperformed sulfonic acid resins (Amberlyst®70, Amberlite®IR120 and LS resin), zeolites and liquid acids (p-toluenesulfonic acid, acetic acid and phenol). In fact, the most active carbocatalyst (60LS40PS350H+) exhibited the same turnover frequency as p-toluenesulfonic acid (186 h−1) upon furfural conversion but with an improved HAA product yield (up to 88%) and reusability, maintaining 98% of its original activity up to seven reaction cycles. The observed catalytic activity and operational stability of the LS derived acidic carbocatalysts were attributed to the strongly Brønsted acidic –SO3H groups covalently incorporated into their structural carbon framework and the promotional effects of hydrophilic surface functional groups (–COOH and –OH) favoring adsorption of oxygenated reactant molecules.


 Please wait while we load your content...
Please wait while we load your content...