Understanding the oxidative dehydrogenation of ethyl lactate to ethyl pyruvate over vanadia/titania†
Abstract
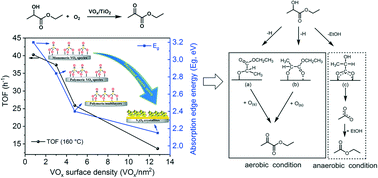
We studied the vapour-phase oxidative dehydrogenation (ODH) of ethyl lactate with air to give ethyl pyruvate over V2O5/TiO2 catalysts in a fixed-bed reactor. The nature of the vanadia species is changed by varying the vanadium surface density, and the corresponding structure of the VOx species was determined by XRD, UV-vis spectroscopy, XPS and H2-TPR. Monomeric and isolated vanadia species dominate at lower vanadium surface densities. As the surface density increases, two-dimensional polyvanadates and bulk-like vanadia crystallites become predominant. The activity per vanadium decreases with increasing vanadium surface density, indicating that the monomeric VOx species is better for pyruvate production and that the V–O–Ti bonds play an important role in the ODH of ethyl lactate. This is also confirmed by the superior catalytic performance of V2O5/TiO2 compared to vanadium supported on MgO, Al2O3, ZrO2 and CeO2. In situ DRIFT spectroscopy coupled with mass analysis shows that the reaction can involve three possible adsorption modes of ethyl lactate on the V2O5/TiO2 surface. Under anaerobic conditions, 2-hydroxypropionate forms, giving ethyl acetate as the major product. Conversely, under aerobic conditions, oxygen that is chemisorbed on V2O5/TiO2 is active and easily replenished from the gas phase, converting the ethyl-propionate-2-oxide intermediate into ethyl pyruvate.


 Please wait while we load your content...
Please wait while we load your content...