Improved thermal stability of a copper-containing ceria-based catalyst for low temperature CO oxidation under simulated diesel exhaust conditions†
Abstract
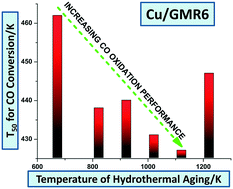
Cerium–zirconium mixed oxides (CZ) containing Cu (CZCu) have very high CO oxidation performance at low temperatures under simulated diesel exhaust conditions; however, CZCu is prone to significant deactivation upon hydrothermal aging. In this work, we discovered that Cu supported on commercial CZ (GMR6) is thermally stable, and after aging Cu/GMR6 shows comparable activity for CO oxidation to the fresh CZCu. GMR6 is more thermally stable than homemade CZ, and Cu/GMR6 shows a smaller decrease of BET surface area than Cu/CZ upon hydrothermal aging. The decrease of the BET surface area of the catalyst is accelerated by the presence of Cu. The presence of La and Pr in the GMR6 support provides high structural stability and enhanced oxygen mobility. The formation of both surface and bulk carbonates during CO oxidation results in the activity decrease of Cu/GMR6. Thermal decomposition of these carbonates fully regenerates Cu/GMR6. The presence of low levels of SO2 in the feed gas mixture poisons the CO oxidation activity of Cu/GMR6 forming highly stable sulfates on the CZ support.


 Please wait while we load your content...
Please wait while we load your content...