Nickel oxide–polypyrrole nanocomposite electrode materials for electrocatalytic water oxidation†
Abstract
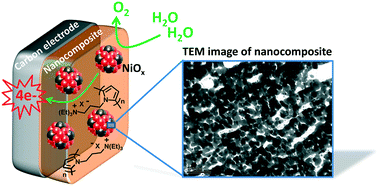
An efficient nanocomposite anode material for the oxygen evolution reaction (OER) based on nickel oxide nanoparticles entrapped in a polypyrrole matrix has been readily synthesized by an all-electrochemical procedure. Examples of anodes for the OER utilizing metallic nanoparticles dispersed in an organic polymer film are still scarce but very promising for water splitting applications. Herein, nickel metal (Ni0) nanoparticles were first electrodeposited into a cationic poly(pyrrole-ammonium) film (poly1) electropolymerized onto carbon or ITO electrodes by reduction of an anionic nickel oxalate complex in aqueous borate buffer at pH 6. The Ni0 nanoparticles were then electro-oxidized into nickel oxide (NiOx) in aqueous borate buffer at pH 9.2. The physical, chemical and electrocatalytic properties of the nanostructured poly1–NiOx material were evaluated by inductively coupled plasma mass spectroscopy (ICP-MS), atomic force microscopy (AFM) and transmission electron microscopy (TEM) coupled to various electrochemical techniques, and the results were compared with those of NiOx directly deposited on a naked electrode (without polymer) by the same electrochemical procedure. Such characterization clearly evidences the beneficial role of the poly1 matrix to generate smaller and non-agglomerated NiOx particles. Owing to the small size of NiOx nanoparticles (ca. 21 nm) and the great nanostructuration of the poly1–NiOx composite film deposited on the carbon (C) electrode surface, this material displays a very high electrocatalytic OER performance in a nearly neutral solution (pH 9.2) and even in alkaline solution (pH 14) with strong mass activities and turnover frequencies (TOFs) (1.12 A mg−1 and 0.17 s−1, respectively, at an overpotential of 0.61 V at pH 9.2, and of 1.28 A mg−1 and 0.19 s−1, respectively, at an overpotential of 0.35 V at pH 14). This performance places the C/poly1–NiOx electrode among the most active anodes based on nickel oxide reported in the literature and undoped with iron ions for the OER. In addition, when the poly1–NiOx film is deposited on a porous ITO electrode, its physisorption on the electrode surface is significantly enhanced, as shown by long-term electrocatalysis at 1.2 V vs. Ag/AgCl. Indeed, its catalytic current remains constant after several days of electrocatalysis, demonstrating the great stability of the poly1–NiOx nanocomposite; the polymer matrix maintains the integrity of the NiOx nanoparticles and precludes their corrosion. This material is thus very promising for the implementation of efficient and highly stable anodes for water oxidation.


 Please wait while we load your content...
Please wait while we load your content...