Active Pd(ii) complexes: enhancing catalytic activity by ligand effect for carbonylation of methyl nitrite to dimethyl carbonate†
Abstract
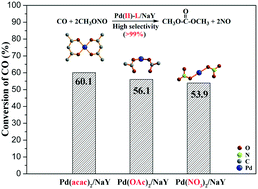
Palladium (Pd)-based catalysts have been widely used for carbonylation of methyl nitrite to dimethyl carbonate (DMC), but a high-performance chloride free catalyst combining both excellent carbon monoxide (CO) conversion and DMC selectivity has not been developed yet. In this work, a chloride free, Pd-based catalyst with good activity and selectivity (conversion of CO: 60.1%, selectivity to DMC: 99.9%) has been successfully fabricated. By thorough characterization and analysis, it is found that the good catalytic activity is positively correlated with the high oxidation states of the Pd species, which could be tuned by their ability to accept the backdonation electron of the ligands. The strong electron backdonation from Pd to π* antibonding orbitals of the ligand in the palladium acetylacetonate [Pd(acac)2] complex accelerates the step where Pdδ+ reoxidizes to Pd(II), resulting in the higher catalytic activity. In addition, a catalytic mechanism was proposed based on the results of X-ray photoelectron spectroscopy and in situ diffuse reflectance infrared spectroscopy. This work not only explains the positive relationship between the catalytic activity and the oxidation state of the Pd species, but also provides a new way to enhance catalytic performance by utilizing the abilities of accepting the backdonation electron of the ligands.


 Please wait while we load your content...
Please wait while we load your content...