On the role of the alkali cations on methanol thiolation†
Abstract
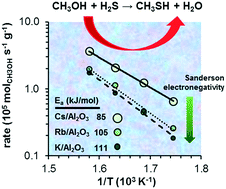
The electronegativity effect of the alkali cations on the formation of methanethiol by reaction of methanol and H2S was studied with K+, Rb+, and Cs+ supported on γ-Al2O3. Catalyst activation in H2S led to the formation of sulfur oxyanions, including SO3−2, S2O3−2, SO4−2 and S2O6−2. The surface modified aluminas have strong basic properties. Accessible alkali cations on γ-Al2O3 are the active sites catalyzing the SN2 nucleophilic substitution. These sites stabilize two Lewis acid–base pairs, formed upon H2S and methanol dissociation, in proximity. Decreasing electronegativity of the alkali enhances the concentration of basic sites and their adsorption capacity. In consequence, the rates of methanethiol formation increased in the sequence K/γ-Al2O3 < Rb/γ-Al2O3 < Cs/γ-Al2O3. The activation energies also decreased in parallel with the decreasing electronegativity of the alkali, which is attributed to the increasing electron density at the SH− groups that facilitates the nucleophilic attack.


 Please wait while we load your content...
Please wait while we load your content...