Tandem catalytic synthesis of benzene from CO2 and H2†
Abstract
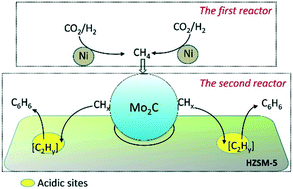
Benzene as an important raw material for production of industrial chemicals is generally synthesized from petroleum and coal tar. Here, we realized the synthesis of benzene from greenhouse CO2 and H2 with two connected reactors by a tandem catalysis reaction comprising CO2 methanation and CH4 aromatization. The Ni/SiO2 catalyst loaded in the first reactor was used to convert CO2 to CH4, and the formed CH4 was sequentially converted to benzene on the Mo/HZSM-5 catalyst in the second reactor. The benzene formation rate reaches 0.68 μmol g−1 min−1 accompanied by a high CO2 conversion of 92%. This concept will provide a new pathway for the direct synthesis of benzene and CO2 utilization.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...