Mechanistic insights into complete hydrogenation of 1,3-butadiene over Pt/SiO2: effect of Pt dispersion and kinetic analysis
Abstract
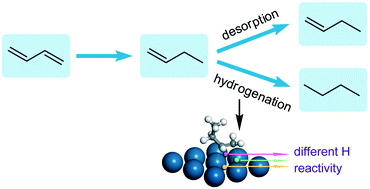
Gas-phase hydrogenation of 1,3-butadiene was investigated over Pt/SiO2 catalysts with the aim of understanding the complete hydrogenation of dienes. The measurements focused on the effect of Pt dispersion on the product distribution and the kinetics of n-butane formation. Under the investigated conditions with an excess of hydrogen, the selectivity to n-butane sharply decreased from 41.6% to less than 3% at a 1,3-butadiene conversion of ∼10% when the average Pt particle size increased from 3.9 to 22 nm. Correspondingly, the apparent activation energy for n-butane formation was measured to increase by about 2.7 times. In situ diffuse reflectance infrared Fourier transform (DRIFT) observations via alternating 1,3-butadiene and hydrogen feeds over the catalysts demonstrated that 1-butene was the major reaction intermediate in the process of 1,3-butadiene hydrogenation. By analysis of the kinetic data using the Horiuti–Polanyi mechanism with 1-butene as the intermediate, different reactivities of hydrogen on the catalysts towards C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond hydrogenation were proposed to understand the variation of the selectivity to n-butane with Pt dispersion. This viewpoint was further supported by DFT calculation of 1-butene hydrogenation with hydrogen at different sites on the Pt(111) surface.
C bond hydrogenation were proposed to understand the variation of the selectivity to n-butane with Pt dispersion. This viewpoint was further supported by DFT calculation of 1-butene hydrogenation with hydrogen at different sites on the Pt(111) surface.
- This article is part of the themed collection: 2017 Catalysis Science & Technology HOT Articles


 Please wait while we load your content...
Please wait while we load your content...