Photocatalytic decomposition of benzene enhanced by the heating effect of light: improving solar energy utilization with photothermocatalytic synergy†
Abstract
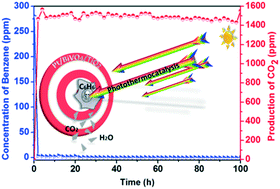
In the photocatalytic oxidation of benzene over BiVO4/TiO2, some deactivation was observed at 30 °C, while stable activity was maintained at higher temperatures. To further take advantage of the heating effect during photocatalysis, Pt was loaded onto the BiVO4/TiO2 sample, owing to its excellent performance in thermocatalysis. The results show that the Pt/BiVO4/TiO2 sample with a Pt loading amount of 1.0 wt% has the best activity in the oxidation of benzene, compared with others containing loading amounts of 0.2, 0.5 and 2.0 wt%. Benzene can be totally oxidized at 80 °C with an obvious photothermocatalytic synergetic effect, whose mechanism is discussed and summarized as three main paths. In this case, the heating effect of infrared light from a light source can be helpful in photocatalysis, which is an inspiration for increasing the availability of solar energy.


 Please wait while we load your content...
Please wait while we load your content...