Tuning electronic states of catalytic sites enhances SCR activity of hexagonal WO3 by Mo framework substitution†
Abstract
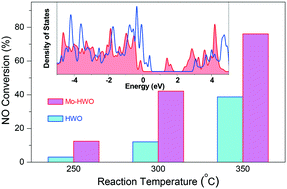
Selective catalytic reduction (SCR) of NO with NH3 essentially proceeds via redox cycles, in which electrons transfer between reactants and catalytically active sites (CASs). Hence, the electronic states of CASs play a crucial role in determining the SCR activity of catalysts. Herein, we tune the electronic structures of CASs via substitution of the hexagonal WO3 framework by Mo (Mo–HWO). The resulting Mo–HWO catalyst shows a high NO conversion of 80% at 350 °C at a gas hourly space velocity of 32 000 h−1, in the presence of high-concentration SO2 (2700 mg m−3) and 10 vol% H2O. Various characterization results demonstrate that the CASs responsible for NH3 adsorption and activation are located at the tunnel openings, i.e., the (001) top-facets of Mo–HWO nanorods. The framework substitution by Mo reduces the bandgap between the highest occupied molecular orbitals and the lowest unoccupied molecular orbitals by hybridizing W and Mo cations with their bridging oxygen ions, thus making the electron transfers in SCR redox cycles relatively easy and leading to improved catalytic activity. This work could assist the rational design of catalysts by tuning the electronic states of CASs.


 Please wait while we load your content...
Please wait while we load your content...