Magnetic CoPt nanoparticle-decorated ultrathin Co(OH)2 nanosheets: an efficient bi-functional water splitting catalyst†
Abstract
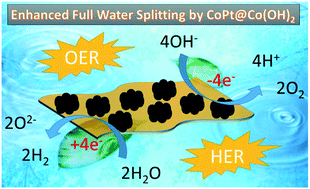
Hydrogen generation via electrocatalytic water splitting is on the cutting edge of energy research. The kinetic burdens associated with the sluggish anodic oxygen evolution reaction (OER) and cathodic hydrogen evolution reaction (HER) cause a large amount of energy loss. Hence, these reactions must be catalyzed. Therefore, an array of magnetic CoPt nanoparticle (NP)-decorated ultrathin Co(OH)2 nanosheets was synthesized and applied for electrocatalytic water splitting in 1 M KOH. CoPt@Co(OH)2 required a minimum overpotential of 334 mV at 10 mA cm−2 in the OER and 226 mV at 50 mA cm−2 in the HER in addition to possessing exceptional stability upon cycling and chronoamperometry. The advantages of the magnetic property of CoPt@Co(OH)2 have been utilized for the first time to improve its stability. The aging study carried out at the CoPt@Co(OH)2-modified interface with and without magnetic support has shown that the magnetically co-stabilized interface was more stable even after 5 days of aging in 1 M KOH. This magnetism-assisted enhancement in the stability of a nanocatalyst-modified interface along with proper further developments will surely take the electrocatalysis of water splitting to a new level.


 Please wait while we load your content...
Please wait while we load your content...