Hexagonal Zn1−xCdxS (0.2 ≤ x ≤ 1) solid solution photocatalysts for H2 generation from water†
Abstract
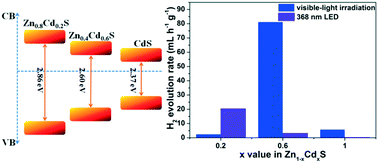
A series of hexagonal Zn1−xCdxS photocatalysts with variable composition (0.2 ≤ x ≤ 1) is synthesized by a solvothermal method. XPS spectra of Zn 2p and Cd 3d regularly shift upon changing the composition of Zn1−xCdxS samples, due to the difference in the Mulliken atomic charges of Zn, Cd, and S. The photocatalytic activity of the samples for H2 evolution from water was tested under visible-light irradiation (λ ≥ 420 nm) without a cocatalyst; the Zn0.4Cd0.6S sample exhibited the highest photocatalytic H2 evolution rate, which was about 81 mL h−1 g−1. Under irradiation with a 368 nm LED, the H2 evolution rate decreased with increasing Cd fraction in the Zn1−xCdxS solid solution. By comparing the crystallinity, surface area, valence/conduction band positions, absorption spectra and fluorescence lifetimes, we conclude that the photocatalytic activity is related to the balance between the absolute positions of the valence/conduction bands and light absorption ability of the Zn1−xCdxS photocatalysts.


 Please wait while we load your content...
Please wait while we load your content...