Selective corrosion of LaCoO3 by NaOH: structural evolution and enhanced activity for benzene oxidation
Abstract
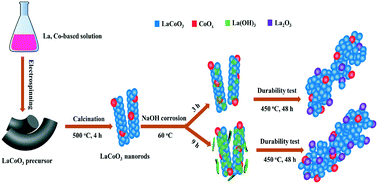
La(OH)3 nanosheets have been successfully coated on electrospun LaCoO3 nanorods using a simple and facile corrosion process, where La cations are selectively precipitated using NaOH. During the oxidation process, La(OH)3 is converted to La2O3 having a mutual effect with the spontaneously generated Co3O4 and the predominant LaCoO3 component. The performances for catalytic benzene oxidation and characterization results indicate that the alkaline treatment of electrospun LaCoO3 can improve the apparent catalytic activity because more surface adsorbed oxygen species and exposed Co3+ are generated. However, if the treatment time reaches 9 h, over-deposition of La(OH)3/La2O3 species on the surface may cause a large fraction of active Co species to be inaccessible, resulting in a lower specific activity (2.9 × 10−6 molC6H6 m−2 h−1) compared to that of untreated LaCoO3 (4.8 × 10−6 molC6H6 m−2 h−1). A treatment time of 3 h provides the highest specific activity, 1.01 × 10−5 molC6H6 m−2 h−1. Moreover, the obtained catalyst shows deactivation of only 3% benzene conversion in a stability test at 450 °C for 48 h. Therefore, NaOH-treated LaCoO3 is a promising catalyst for the practical removal of volatile organic compounds due to its high efficiency, good stability, and convenient preparation.


 Please wait while we load your content...
Please wait while we load your content...