Hydrodeoxygenation of gamma-valerolactone on transition metal phosphide catalysts†
Abstract
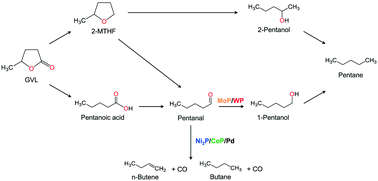
The catalytic hydrodeoxygenation (HDO) of the cyclic five-membered ester gamma-valerolactone (GVL-C5H8O2) on a series of supported metal phosphide catalysts and a commercial Pd/Al2O3 catalyst was studied at 0.5 MPa. Comparison of activities was based on turnover frequencies calculated from surface metal atoms determined from the chemisorption of CO at 50 °C. It was found that catalytic activity followed the order: Ni2P/MCM-41 ≫ CoP/MCM-41 ≫ Pd/Al2O3 ≈ MoP/MCM-41 > WP/MCM-41. On all catalysts, ring opening of the lactone to produce pentanoic acid was the main initial step with subsequent hydrogenation to form pentanal. On Ni2P/MCM-41, CoP/MCM-41 and Pd/Al2O3, this was followed mainly by decarbonylation to produce CO and saturated C4 hydrocarbons. On MoP/MCM-41 and WP/MCM-41, the principal subsequent step was deoxygenation to produce unsaturated C5 hydrocarbons. A possible reaction network is proposed based on product selectivity and selectivity versus conversion plots.

 Please wait while we load your content...
Please wait while we load your content...