Kinetic evidence: the rate-determining step for ammonia synthesis over electride-supported Ru catalysts is no longer the nitrogen dissociation step†
Abstract
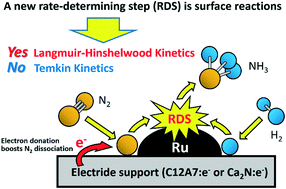
Using kinetic analysis, we demonstrate that the rate-determining step for ammonia synthesis over Ru catalysts supported by electrides, such as [Ca24Al28O64]4+(e−)4 and Ca2N:e−, is not the N2 dissociation step but the subsequent surface reactions of N and H adatoms, in which case the Langmuir–Hinshelwood model should be used to describe the kinetics.

 Please wait while we load your content...
Please wait while we load your content...