Reaction mechanism of dimethyl ether carbonylation to methyl acetate over mordenite – a combined DFT/experimental study
Abstract
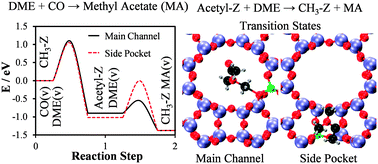
The reaction mechanism of dimethyl ether carbonylation to methyl acetate over mordenite was studied theoretically with periodic density functional theory calculations including dispersion forces and experimentally in a fixed bed flow reactor at pressures between 10 and 100 bar, dimethyl ether concentrations in CO between 0.2 and 2.0%, and at a temperature of 438 K. The theoretical study showed that the reaction of CO with surface methyl groups, the rate-limiting step, is faster in the eight-membered side pockets than in the twelve-membered main channel of the zeolite; the subsequent reaction of dimethyl ether with surface acetyl to form methyl acetate was demonstrated to occur with low energy barriers in both the side pockets and in the main channel. The present analysis has thus identified a path, where the entire reaction occurs favourably on a single site within the side pocket, in good agreement with previous experimental studies. The experimental study of the reaction kinetics was consistent with the theoretically derived mechanism and in addition revealed that the methyl acetate product inhibits the reaction – possibly by sterically hindering the attack of CO on the methyl groups in the side pockets.


 Please wait while we load your content...
Please wait while we load your content...