Oxygen reduction reaction at LaxCa1−xMnO3 nanostructures: interplay between A-site segregation and B-site valency†
Abstract
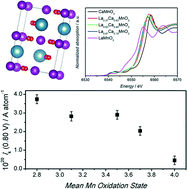
The mean activity of surface Mn sites at LaxCa1−xMnO3 nanostructures towards the oxygen reduction reaction (ORR) in alkaline solution is assessed as a function of the oxide composition. Highly active oxide nanoparticles were synthesised by an ionic liquid-based route, yielding phase-pure nanoparticles, across the entire range of compositions, with sizes between 20 and 35 nm. The bulk vs. surface composition and structure are investigated by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS) and X-ray absorption near edge spectroscopy (XANES). These techniques allow quantification of not only changes in the mean oxidation state of Mn as a function of x, but also the extent of A-site surface segregation. Both trends manifest themselves in the electrochemical responses associated with surface Mn sites in 0.1 M KOH solution. The characteristic redox signatures of Mn sites are used to estimate their effective surface number density. This parameter allows comparing, for the first time, the mean electrocatalytic activity of surface Mn sites as a function of the LaxCa1−xMnO3 composition. The ensemble of experimental data provides a consistent picture in which increasing electron density at the Mn sites leads to an increase in the ORR activity. We also demonstrate that normalisation of electrochemical activity by mass or specific surface area may result in inaccurate structure–activity correlations.

 Please wait while we load your content...
Please wait while we load your content...