Selective catalytic reduction of nitric oxide with ammonia over high-activity Fe/SSZ-13 and Fe/one-pot-synthesized Cu-SSZ-13 catalysts
Abstract
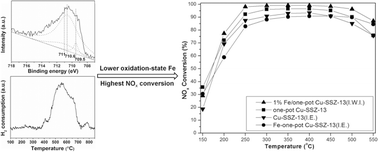
Fe/SSZ-13 and Fe/one-pot-synthesized Cu-SSZ-13 catalysts were prepared by a simple incipient wetness impregnation (I.W.I.) method. These Fe-impregnated catalysts showed higher catalytic activities for the selective catalytic reduction (SCR) of NO with ammonia as compared to other high-activity catalysts that were prepared by the ion-exchange (I.E.) method, such as Fe-SSZ-13, Cu-SSZ-13 and one-pot-synthesized Cu-SSZ-13. These Fe-impregnated catalysts also exhibited higher SO2 resistance and yielded lower N2O product concentrations. The catalysts were characterized by XRD, XPS, ESR, H2-TPR and ICP. The XPS and H2-TPR results indicated that the lower oxidation-state Fe which existed in the Fe-impregnated sample via incipient wetness impregnation was beneficial for the NH3-SCR activity.

 Please wait while we load your content...
Please wait while we load your content...