Carbonyl group-dependent high-throughput screening and enzymatic characterization of diaromatic ketone reductase†
Abstract
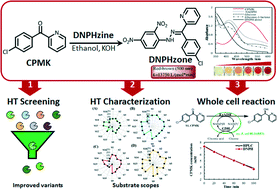
We have developed a carbonyl group-dependent colorimetric method for assay of carbonyl reductases using inexpensive 2,4-dinitrophenylhydrazine (DNPH). This DNPH method has high sensitivity and low background disturbance, and can be used for high-throughput screening of carbonyl reductases toward various ketones including diaromatic ketones, aliphatic ketones/diketones, and aromatic ketones. For 1-(4′-chlorophenyl)-1-(pyridine-2′-yl)-methyl ketone (CPMK), a characteristic absorbance peak at around 500 nm was observed for the product of CPMK and DNPH with a molar absorbance coefficient of 13 750 L mol−1 cm−1. Significantly, this method is also amendable to whole-cell systems for facile high-throughput screening and substrate specificity profiling. In random mutagenesis of a diaromatic ketone reductase KpADH, three variants (M131F, S196Y and S237A) with improved activity toward CPMK were identified using the DNPH method. The substrate specificity of KpADH and its variants toward sixteen prochiral ketones was well characterized. Furthermore, kinetics and molecular docking analyses of KpADH and its variants were performed to elucidate the potential catalytic and enantioselective mechanisms. Consequently, our study provides a rapid screening and characterization method for carbonyl reductases with potential applications in the preparation of optically active secondary alcohols.

 Please wait while we load your content...
Please wait while we load your content...