Unusual effect of cobalt on Cu–MgO catalyst for the synthesis of γ-butyrolactone and aniline via coupling reaction
Abstract
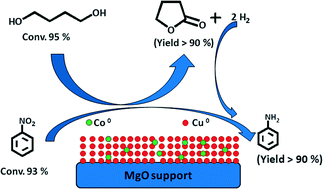
The addition of cobalt to the Cu–MgO catalyst revealed apparent changes in the parent solid structure and texture, which led to noteworthy elevation in both the dehydrogenation of 1,4-butanediol (BDO) and hydrogenation of nitrobenzene (NB) and their simultaneous coupling for the synthesis of γ-butyrolactone (GBL) and aniline (AN). Hence, an attempt is made to unravel the effect of cobalt through the preparation of various cobalt-containing Cu–MgO catalysts (xCo–20Cu–(80 − x)MgO, where x = 1, 5, 10 and 15 wt%) by a hydroxycarbonate gel mixing method. Systematic characterization of these catalysts using AAS analysis, N2 physisorption, X-ray diffraction, H2 temperature-programmed reduction, N2O pulse chemisorption and X-ray photoelectron spectroscopy was carried out. N2O pulse chemisorption confirms the presence of a larger metal surface area, dispersion and surface coverage with a smaller particle size of Co–Cu. 10Co–20Cu–70MgO with a smaller Cu particle size was found to be the best catalyst among the series of Co–Cu–MgO catalysts, in terms of activity in the simultaneous coupling of BDO dehydrogenation and NB hydrogenation with >90% yield of GBL and AN, respectively.

 Please wait while we load your content...
Please wait while we load your content...