Oxidation of chlorinated alkanes over Co3O4/SBA-15 catalysts. Structural characterization and reaction mechanism†
Abstract
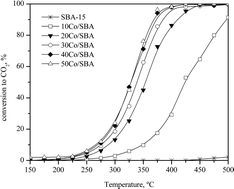
Cobalt oxide-based catalysts have been synthesised via a hard template of mesoporous silica SBA-15 in the form of massive Co3O4 nanoparticles, whose dimensions are controlled and limited by the support mesoporosity. Over this family of catalysts, both weak Brønsted and Lewis acidities have been detected, with the relative abundance a function of the cobalt content. The mesoporosity of the support leads to the growth of oxide nanoparticles, mainly in the small pores, thus improving their redox properties. Catalysts possessing cobalt oxide loadings higher than 30% present an adequate activity for the deep DCE oxidation toward carbon dioxide, hydrogen chloride and chlorine. It is believed that the reaction is markedly accelerated due to the simultaneous participation of the acid sites (where the chlorinated feed is efficiently adsorbed) and the redox sites (oxidation of the adsorbed feed with lattice oxygen anions). FTIR data on dichloroalkane oxidation evidence that lattice oxygen species are mainly involved in the Cl-VOC combustion, likely through a Mars–van Krevelen mechanism.

 Please wait while we load your content...
Please wait while we load your content...