Mechanistic understanding of ternary Ag/AgCl@La(OH)3 nanorods as novel visible light plasmonic photocatalysts
Abstract
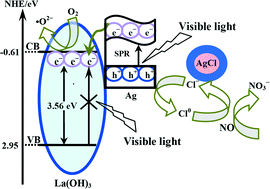
Ternary Ag/AgCl@La(OH)3 (Ag/AgCl/La) composite photocatalysts were prepared by a facile chemical method. The enhanced visible-light absorption and charge carrier separation can be simultaneously realized after the introduction of Ag/AgCl particles into La(OH)3 nanorods. The Ag/AgCl/La composites were applied to the visible-light photocatalytic oxidization of NO in air and exhibited an enhanced activity for NO removal in comparison with Ag/AgCl and pure La(OH)3 nanorods. The photocatalytic performance of Ag/AgCl/La was dependent on the mass ratio of Ag/AgCl to La(OH)3. The highest photocatalytic performance can be achieved when the mass ratio of Ag/AgCl to La(OH)3 is controlled at 1 : 2. Further increasing the mass ratio of Ag/AgCl promoted the aggregation of Ag/AgCl particles, which was not beneficial to improving the surface plasmon resonance effects. A new photocatalytic mechanism for Ag/AgCl/La was proposed, which was related to the surface plasmon resonance effects of the Ag metal and the effective carrier separation ability of La(OH)3. Importantly, the as-prepared Ag/AgCl/La composites exhibited high photochemical stability after multiple reaction runs. The concept of enhancing the activity of a semiconductor with a wide band gap through the SPR effects of Ag/AgCl could provide new approaches for the development of efficient plasmonic photocatalysts.

 Please wait while we load your content...
Please wait while we load your content...