Synthesis and high electrocatalytic activity of Au-decorated Pd heterogeneous nanocube catalysts for ethanol electro-oxidation in alkaline media
Abstract
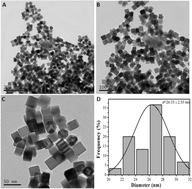
In this study, highly active Au-decorated Pd heterogeneous nanocubes with Pd/Au molar ratios ranging from 15 : 1 to 2 : 1 were successfully synthesized based on a successive reduction strategy. The structure, morphology and composition of the as-prepared catalysts were characterized by X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), transmission electron microscopy (TEM), high-resolution TEM (HR-TEM), energy dispersive spectrometer (EDS) line scan and the elemental mapping. The results show that the lattice orientations of the Pd nanocubes match those of the Au layers. Structural analysis establishes that the surface of the Au-decorated Pd nanocrystal shows its {111} faces. The heterogeneous nanocubes were used for ethanol electro-oxidation reaction in alkaline media. The electrochemical results indicate that the addition of Au to Pd can significantly improve the performance including catalytic activity, CO tolerance and stability. These results clearly suggest that the relative amounts of Au and Pd on the surface of the nanocubes are crucial for the improvement of Pd catalysis, and Pd5Au1 is identified as the most efficient catalyst since it possesses superior catalytic activity and long-term stability.

 Please wait while we load your content...
Please wait while we load your content...