Influence of adsorption strength in aqueous phase glycerol hydrodeoxygenation over Ni@Pt and Co@Pt overlayer catalysts
Abstract
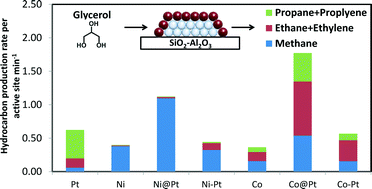
Silica–alumina supported bimetallic overlayer catalysts of platinum on nickel (Ni@Pt) and platinum on cobalt (Co@Pt) were synthesized using the directed deposition technique. Hydrogen chemisorption and an ethylene hydrogenation descriptor reaction were used to study the adsorption strength of platinum overlayer compared to pure platinum. Decreased hydrogen heats of adsorption from H2 chemisorption were observed for overlayer catalysts compared to pure platinum, consistent with computational predictions. Ethylene hydrogenation reactivity decreased for overlayer catalysts compared to pure Pt which also implies weakened hydrogen binding of overlayer catalysts. In aqueous phase glycerol hydrodeoxygenation, overlayer catalysts showed higher hydrocarbon production rates per active site compared to their monometallic counterparts and bimetallic alloy samples, likely due to the decreased adsorption strength on Pt overlayer so that less Pt active sites were blocked by strongly adsorbed species such as H2 and CO. For reactions where strong product binding has been shown to inhibit the reaction, the weakened binding associated with the overlayer catalysts should result in increased activity.

 Please wait while we load your content...
Please wait while we load your content...