Kinetic studies of fluorinated aryl molybdenum(ii) tricarbonyl precursors in epoxidation catalysis†
Abstract
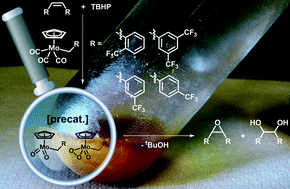
Benzyl substituted molybdenum tricarbonyl complexes displaying CF3 groups are synthesized and applied as catalyst precursors in olefin epoxidation reactions. The CF3 moiety is important for both decarboxylation velocity and active species formation. DFT calculations as well as 1H, 13C, 19F and 95Mo NMR spectroscopy help explain the observed reactivity differences. A variety of olefins can be epoxidized and TOFs of up to ca. 22 000 h−1 are obtained in hexafluoroisopropanol (HFIP). Furthermore, it is possible to recycle the active species at least 10 times without significant activity loss in a two-phase catalytic reaction. 19F NMR kinetic studies reveal that at least two intermediates are formed during the reaction with excess TBHP, depending on the position of the CF3 group. The substrate addition mode has also a major influence on the catalyst formation velocity.

 Please wait while we load your content...
Please wait while we load your content...