The curious effects of integrating bimetallic active centres within nanoporous architectures for acid-catalysed transformations†
Abstract
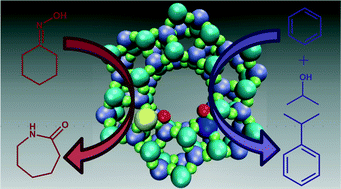
The resourceful combination of distinct Mg, Zn and Si active-sites within a single aluminophosphate framework, via simultaneous isomorphous substitution, has afforded unique bimetallic nanoporous heterogeneous catalysts. Unique site-specific interactions have been engineered, at the molecular level, to facilitate catalytic modifications and optimize product yield. By the dextrous incorporation of individual transition-metal active centres, we are able to intricately control the precise nature of the Brønsted acid sites, thereby influencing their catalytic behaviour for the industrially relevant acid-catalysed Beckmann rearrangement of cyclohexanone oxime and isopropylation of benzene.
- This article is part of the themed collection: Catalysis on Zeolites

 Please wait while we load your content...
Please wait while we load your content...