Ketone hydrogenation catalyzed by a new iron(ii)–PNN complex†
Abstract
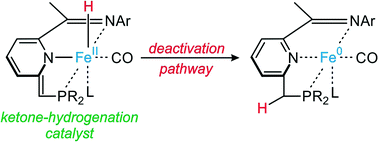
The formal FeII–hydride complex [Fe(H)(CO)(MeCN)LPNN](BF4) (1) (LPNN = 2-[(di-tert-butylphosphino)methyl]-6-[1-(mesitylimino)ethyl]pyridine) catalyzes the hydrogenation of ketones under mild conditions (room temperature, p(H2) = 4 bar) and short reaction times (1–3 h) in the presence of catalytic amounts of KHMDS as a base. The reaction presumably proceeds via a dearomatization/rearomatization mechanism. However, in comparison with the reaction of related iron–PNP complexes, the reaction mechanism seems to be different, and an enolate formation step appears to precede catalysis. Moreover, the catalytic performance of the PNN system is inferior under similar conditions, and this observation is probably a consequence of an intramolecular deactivation pathway, which involves reductive proton migration within a dearomatized FeII–hydride complex to form a catalytically inactive Fe0 species. The weaker electron-donating properties of the PNN ligand system, when compared with analogous PNP-based ligands, cause the dearomatized PNN iron(II)–hydride intermediate to be less electron-rich and consequently more prone to the intramolecular reductive elimination pathway. This result is in line with the need for electron-rich metal hydrides for efficient hydrogenation catalysis to take place.

 Please wait while we load your content...
Please wait while we load your content...