Microwave-assisted synthesis of porous Mn2O3 nanoballs as bifunctional electrocatalyst for oxygen reduction and evolution reaction†
Abstract
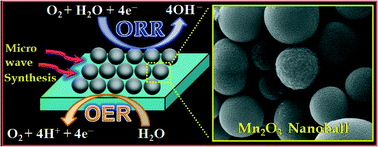
Technological hurdles that still prevent the commercialization of fuel cell technologies necessitate designing low-cost, efficient and non-precious metals. These could serve as alternatives to high-cost Pt-based materials. Herein, a facile and effective microwave-assisted route has been developed to synthesize structurally uniform and electrochemically active pure and transition metal-doped manganese oxide nanoballs (Mn2O3 NBs) for fuel cell applications. The average diameter of pure and doped Mn2O3 NBs was found to be ~610 nm and ~650 nm, respectively, as estimated using transmission electron microscopy (TEM). The nanoparticles possess a good degree of crystallinity as evident from the lattice fringes in high-resolution transmission electron microscopy (HRTEM). The cubic crystal phase was ascertained using X-ray diffraction (XRD). The energy dispersive spectroscopic (EDS) elemental mapping confirms the formation of copper-doped Mn2O3 NBs. The experimental parameter using trioctylphosphine oxide (TOPO) as the chelating agent to control the nanostructure growth has been adequately addressed using scanning electron microscopy (SEM). The solid NBs were formed by the self-assembly of very small Mn2O3 nanoparticles as evident from the SEM image. Moreover, the concentration of TOPO was found to be the key factor whose subtle variation can effectively control the size of the as-prepared Mn2O3 NBs. The cyclic voltammetry and galvanostatic charge/discharge studies demonstrated enhanced electrochemical performance for copper-doped Mn2O3 NBs which is supported by a 5.2 times higher electrochemically active surface area (EASA) in comparison with pure Mn2O3 NBs. Electrochemical investigations indicate that both pure and copper-doped Mn2O3 NBs exhibit a bifunctional catalytic activity toward the four-electron electrochemical reduction as well the evolution of oxygen in alkaline media. Copper doping in Mn2O3 NBs revealed its pronounced impact on the electrocatalytic activity with a high current density for the electrochemical oxygen reduction and evolution reaction. The synthetic approach provides a general platform for fabricating well-defined porous metal oxide nanostructures with prospective applications as low-cost catalysts for alkaline fuel cells.
- This article is part of the themed collection: 2016 most accessed Catalysis Science and Technology articles

 Please wait while we load your content...
Please wait while we load your content...