NHPI and ferric nitrate: a mild and selective system for aerobic oxidation of benzylic methylenes†
Abstract
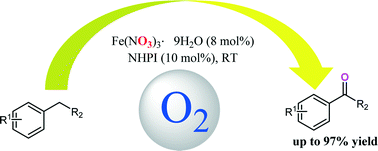
A mild and selective system comprising N-hydroxyphthalimide (NHPI) and Fe(NO3)3·9H2O was developed for the oxidation of benzylic methylenes with an atmospheric pressure of molecular oxygen at 25 °C. The influences of reaction conditions such as solvent, different metal catalysts and catalyst loading were studied, as well as the kinetics of the oxidation reaction. Various benzylic methylene substrates could be oxidized to the corresponding carbonyl compounds in satisfactory yields with this catalytic system. Hammett analysis suggested that the substrates with electron-donating groups would have higher oxidation rates. Isotopic (18O) labeling experiments provided evidence of the participation of the nitrate anion in the catalytic cycle. In addition, a possible radical mechanism involving hydrogen atom abstraction by PINO (phthalimide-N-oxyl) and nitrate participation for the oxidation of benzylic methylenes in the Fe(NO3)3·9H2O/NHPI/O2 system was proposed.

 Please wait while we load your content...
Please wait while we load your content...