Synthetic natural gas by direct CO2 hydrogenation on activated takovites: effect of Ni/Al molar ratio
Abstract
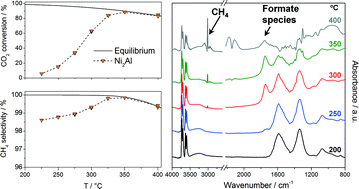
Takovite-derived mixed oxides in Ni/Al molar ratios from 1 to 3 have been used as catalysts in hydrogenation of CO2 to CH4. The catalysts were characterized by XRD, BET, TEM, TGA, and H2-TPR, and monitored by in situ DRIFTS under reaction conditions. The catalytic performance for the CO2 methanation has been investigated in a fixed-bed reactor at the temperature range from 225 to 400 °C and pressures of 10.0 and 1.0 bar(g). Takovite decomposition leads to the formation of a NiO phase containing Al ions and a nickel-containing alumina phase (Ni-deficient spinel). The percentage of spinel increases upon decreasing the Ni/Al ratio, and consequently, a lower amount of metallic nickel after subsequent reduction is achieved. All catalysts were partially reduced upon time on stream, leading to the formation of small Ni0 crystallites (ca. 3 nm) dispersed on a NiAl2O4 matrix. The most active and selective catalyst was the one with a Ni/Al ratio of 2, which was also very stable after a 500 h lifetime test at atmospheric pressure and 275 °C.


 Please wait while we load your content...
Please wait while we load your content...